Citation: Tumiene B, Peters H, Melegh B, Peterlin B, Utkus A, Fatkulina N, Pfliegler G, Graessner H, Hermanns S, Scarpa M, Blay JY, Ashton S, McKay L, Baynam G. Rare disease education in Europe and beyond: time to act. Orphanet journal of rare diseases. 2022; 17(1):441. https://doi.org/10.1186/s13023-022-02527-y.
Citation: Tumiene B, Peters H, Melegh B, Peterlin B, Utkus A, Fatkulina N, Pfliegler G, Graessner H, Hermanns S, Scarpa M, Blay JY, Ashton S, McKay L, Baynam G. Rare disease education in Europe and beyond: time to act. Orphanet journal of rare diseases. 2022; 17(1):441. https://doi.org/10.1186/s13023-022-02527-y.
Publications and Guidelines
Publications
 Citation: Sikonja J, Groselj U, Scarpa M, la Marca G, Cheillan D, Kölker S, Zetterström RH, Kožich V, Le Cam Y, Gumus G, Bottarelli V, van der Burg M, Dekkers E, Battelino T, Prevot J, Schielen PCJI, Bonham JR. Towards Achieving Equity and Innovation in Newborn Screening across Europe. International Journal of Neonatal Screening. 2022; 8(2):31. https://doi.org/10.3390/ijns8020031
Citation: Sikonja J, Groselj U, Scarpa M, la Marca G, Cheillan D, Kölker S, Zetterström RH, Kožich V, Le Cam Y, Gumus G, Bottarelli V, van der Burg M, Dekkers E, Battelino T, Prevot J, Schielen PCJI, Bonham JR. Towards Achieving Equity and Innovation in Newborn Screening across Europe. International Journal of Neonatal Screening. 2022; 8(2):31. https://doi.org/10.3390/ijns8020031
 Citation: Scarpa M, Bonham JR, Dionisi-Vici C, Prevot J, Pergent M, Meyts I, Mahlaoui N, Schielen PCJI. Newborn screening as a fully integrated system to stimulate equity in neonatal screening in Europe. Lancet Reg Health Eur. 2022 Jan 28;13:100311. doi: 10.1016/j.lanepe.2022.100311. PMID: 35199083; PMCID: PMC8841274.
Citation: Scarpa M, Bonham JR, Dionisi-Vici C, Prevot J, Pergent M, Meyts I, Mahlaoui N, Schielen PCJI. Newborn screening as a fully integrated system to stimulate equity in neonatal screening in Europe. Lancet Reg Health Eur. 2022 Jan 28;13:100311. doi: 10.1016/j.lanepe.2022.100311. PMID: 35199083; PMCID: PMC8841274.
Citation: Čechová A, Honzík T, Edmondson AC, Ficicioglu C, Serrano M, Barone R, De Lonlay P, Schiff M, Witters P, Lam C, Patterson M, Janssen MCH, Correia J, Quelhas D, Sykut-Cegielska J, Plotkin H, Morava E, Sarafoglou K. Should patients with Phosphomannomutase 2-CDG (PMM2-CDG) be screened for adrenal insufficiency? Mol Genet Metab. 2021 Aug;133(4):397-399. doi: 10.1016/j.ymgme.2021.06.003. Epub 2021 Jun 11. PMID: 34140212.
 Citation: Brennenstuhl H, Nashawi M, Schröter J, Baronio F, Beedgen L, Gleich F, Jeltsch K, von Landenberg C, Martini S, Simon A, Thiel C, Tsiakas K, Opladen T, Kölker S, Hoffmann GF, Haas D; Unified Registry for Inherited Metabolic Disorders (U-IMD) Consortium and the European Registry for Hereditary Metabolic Disorders (MetabERN). Phenotypic diversity, disease progression, and pathogenicity of MVK missense variants in mevalonic aciduria. J Inherit Metab Dis. 2021 Sep;44(5):1272-1287. doi: 10.1002/jimd.12412. Epub 2021 Jun 28. PMID: 34145613.
Citation: Brennenstuhl H, Nashawi M, Schröter J, Baronio F, Beedgen L, Gleich F, Jeltsch K, von Landenberg C, Martini S, Simon A, Thiel C, Tsiakas K, Opladen T, Kölker S, Hoffmann GF, Haas D; Unified Registry for Inherited Metabolic Disorders (U-IMD) Consortium and the European Registry for Hereditary Metabolic Disorders (MetabERN). Phenotypic diversity, disease progression, and pathogenicity of MVK missense variants in mevalonic aciduria. J Inherit Metab Dis. 2021 Sep;44(5):1272-1287. doi: 10.1002/jimd.12412. Epub 2021 Jun 28. PMID: 34145613.
Citation: Zerjav Tansek M, Kodric J, Klemencic S, Boelens JJ, van Hasselt PM, Drole Torkar A, Doric M, Koren A, Avcin S, Battelino T, Groselj U. Therapy-type related long-term outcomes in mucopolysaccaridosis type II (Hunter syndrome) – Case series. Mol Genet Metab Rep. 2021 Jun 26;28:100779. doi: 10.1016/j.ymgmr.2021.100779. PMID: 34258227; PMCID: PMC8251508.
Citation: Molema F, Martinelli D, Hörster F, Kölker S, Tangeraas T, de Koning B, Dionisi-Vici C, Williams M; additional individual contributors of MetabERN. Liver and/or kidney transplantation in amino and organic acid-related inborn errors of metabolism: An overview on European data. J Inherit Metab Dis. 2021 May;44(3):593-605. doi: 10.1002/jimd.12318. Epub 2020 Oct 29. PMID: 32996606.
Citation: Opladen T, Gleich F, Kozich V, Scarpa M, Martinelli D, Schaefer F, Jeltsch K, Juliá-Palacios N, García-Cazorla Á, Dionisi-Vici C, Kölker S. U-IMD: the first Unified European registry for inherited metabolic diseases. Orphanet J Rare Dis. 2021 Feb 18;16(1):95. doi: 10.1186/s13023-021-01726-3. PMID: 33602304; PMCID: PMC7893973.
Citation: Rossi A, Hoogeveen IJ, Lubout CMA, de Boer F, Fokkert-Wilts MJ, Rodenburg IL, van Dam E, Grünert SC, Martinelli D, Scarpa M; CONNECT MetabERN Collaboration Group, Dekker H, Te Boekhorst ST, van Spronsen FJ, Derks TGJ. A generic emergency protocol for patients with inborn errors of metabolism causing fasting intolerance: A retrospective, single-center study and the generation of www.emergencyprotocol.net. J Inherit Metab Dis. 2021 Apr 12. doi: 10.1002/jimd.12386. Epub ahead of print. PMID: 33844307.
Citation: Stepien KM, Kieć-Wilk B, Lampe C, Tangeraas T, Cefalo G, Belmatoug N, Francisco R, Del Toro M, Wagner L, Lauridsen AG, Sestini S, Weinhold N, Hahn A, Montanari C, Rovelli V, Bellettato CM, Paneghetti L, van Lingen C, Scarpa M. Challenges in Transition From Childhood to Adulthood Care in Rare Metabolic Diseases: Results From the First Multi-Center European Survey. Front Med (Lausanne). 2021 Feb 25;8:652358. doi: 10.3389/fmed.2021.652358. PMID: 33738294; PMCID: PMC7962750.
Citation: Tumiene B, Graessner H, Mathijssen IM, Pereira AM, Schaefer F, Scarpa M, Blay JY, Dollfus H, Hoogerbrugge N. European Reference Networks: challenges and opportunities. J Community Genet. 2021 Apr;12(2):217-229. doi: 10.1007/s12687-021-00521-8. Epub 2021 Mar 17. PMID: 33733400; PMCID: PMC7968406.
Citation: Lampe C, Dionisi-Vici C, Bellettato CM, Paneghetti L, van Lingen C, Bond S, Brown C, Finglas A, Francisco R, Sestini S, Heard JM, Scarpa M; MetabERN collaboration group. The impact of COVID-19 on rare metabolic patients and healthcare providers: results from two MetabERN surveys. Orphanet J Rare Dis. 2020 Dec 3;15(1):341. doi: 10.1186/s13023-020-01619-x. PMID: 33272301; PMCID: PMC7711270.
Citation: Heard JM, Vrinten C, Schlander M, Bellettato CM, van Lingen C, Scarpa M; MetabERN collaboration group. Availability, accessibility and delivery to patients of the 28 orphan medicines approved by the European Medicine Agency for hereditary metabolic diseases in the MetabERN network. Orphanet J Rare Dis. 2020 Jan 6;15(1):3. doi: 10.1186/s13023-019-1280-5. PMID: 31907071; PMCID: PMC6945588.
Guidelines
Citation: Muschol, N., Giugliani, R., Jones, S.A. et al. Sanfilippo syndrome: consensus guidelines for clinical care. Orphanet J Rare Dis 17, 391 (2022). https://doi.org/10.1186/s13023-022-02484-6
 Citation: Hahn A, Lampe C, Boentert M, Hundsberger T, Löscher W, Wenninger S, Ziegler A, Lagler F, Ballhausen D, Schlegel T, Schoser B. Heiminfusionstherapie bei Morbus Pompe: Konsensusempfehlungen für den deutschsprachigen Raum [Home infusion therapy for Pompe disease: Recommendations for German-speaking countries]. Fortschr Neurol Psychiatr. 2021 Dec;89(12):630-636. German. doi: 10.1055/a-1365-8977. Epub 2021 Feb 9. Erratum in: Fortschr Neurol Psychiatr. 2021 Apr 27;: PMID: 33561874.
Citation: Hahn A, Lampe C, Boentert M, Hundsberger T, Löscher W, Wenninger S, Ziegler A, Lagler F, Ballhausen D, Schlegel T, Schoser B. Heiminfusionstherapie bei Morbus Pompe: Konsensusempfehlungen für den deutschsprachigen Raum [Home infusion therapy for Pompe disease: Recommendations for German-speaking countries]. Fortschr Neurol Psychiatr. 2021 Dec;89(12):630-636. German. doi: 10.1055/a-1365-8977. Epub 2021 Feb 9. Erratum in: Fortschr Neurol Psychiatr. 2021 Apr 27;: PMID: 33561874.
 Citation: Schwering C, Kammler G, Wibbeler E, et al. Development of the “Hamburg Best Practice Guidelines for ICV−Enzyme Replacement therapy (ERT) in CLN2 Disease” Based on 6 Years Treatment Experience in 48 Patients. Journal of Child Neurology. 2021;36(8):635-641. doi:10.1177/0883073821989154
Citation: Schwering C, Kammler G, Wibbeler E, et al. Development of the “Hamburg Best Practice Guidelines for ICV−Enzyme Replacement therapy (ERT) in CLN2 Disease” Based on 6 Years Treatment Experience in 48 Patients. Journal of Child Neurology. 2021;36(8):635-641. doi:10.1177/0883073821989154
 Citation: Forny P, Hörster F, Ballhausen D, Chakrapani A, Chapman KA, Dionisi-Vici C, Dixon M, Grünert SC, Grunewald S, Haliloglu G, Hochuli M, Honzik T, Karall D, Martinelli D, Molema F, Sass JO, Scholl-Bürgi S, Tal G, Williams M, Huemer M, Baumgartner MR. Guidelines for the diagnosis and management of methylmalonic acidaemia and propionic acidaemia: First revision. J Inherit Metab Dis. 2021 May;44(3):566-592. doi: 10.1002/jimd.12370. Epub 2021 Mar 9. PMID: 33595124; PMCID: PMC8252715.
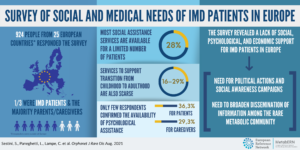
Citation: Forny P, Hörster F, Ballhausen D, Chakrapani A, Chapman KA, Dionisi-Vici C, Dixon M, Grünert SC, Grunewald S, Haliloglu G, Hochuli M, Honzik T, Karall D, Martinelli D, Molema F, Sass JO, Scholl-Bürgi S, Tal G, Williams M, Huemer M, Baumgartner MR. Guidelines for the diagnosis and management of methylmalonic acidaemia and propionic acidaemia: First revision. J Inherit Metab Dis. 2021 May;44(3):566-592. doi: 10.1002/jimd.12370. Epub 2021 Mar 9. PMID: 33595124; PMCID: PMC8252715.The COVID-19 pandemic is testing the resilience of robust health systems around the world. This may be impacting you in many different ways, such as creating additional anxiety or exacerbating other medical or therapy issues related to your Inherited Metabolic Disease (IMD). In this difficult period, MetabERN underlines the critical importance of sustaining efforts to prevent, diagnose and treat Inherited Metabolic Diseases (IMDs) assuring the continuation of the quality of care provided to you. To help the entire metabolic community, we have developed some recommendations that aim to support all rare IMD patients and caregivers during the COVID-19 emergency.
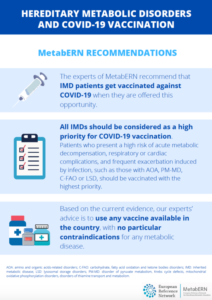
COVID-19 Vaccine Recommendations
The experts of MetabERN recommend that IMD patients get vaccinated against COVID-19 when they are offered this opportunity.
All IMDs should be considered as a high priority for COVID-19 vaccination. Patients who present a high risk of acute metabolic decompensation, respiratory or cardiac complications, and frequent exacerbation induced by infection, such as those with AOA, PM-MD, C-FAO and LSD, should be vaccinated with the highest priority.
Based on the current evidence, our experts’ advice is to use any vaccine available in the country, with no particular contraindications for any metabolic disease.
Find more information here
 Copyright ©2017-2021 all rights reserved
Copyright ©2017-2021 all rights reserved